Enthalpy is defined as the total heat content or total useful energy of a substance. Web the diagram below can be used to determine enthalpy versus entropy of water and steam. Web based on the phase diagram (supplementary fig. Calculate propierties of wet, saturated and superheated steam, steam quality and more. New york, new york, 1979;
Web enthalpy ( / ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. U = specific internal energy. Calculate propierties of wet, saturated and superheated steam, steam quality and more. Web the heat that passes into or out of the system during a reaction is the enthalpy change. Web the diagram below can be used to determine enthalpy versus entropy of water and steam.
Web definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well. Whether the enthalpy of the system increases (i.e. Web enthalpy ( / ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in. The symbol for enthalpy is “h.” enthalpy is also considered to be the sum of.
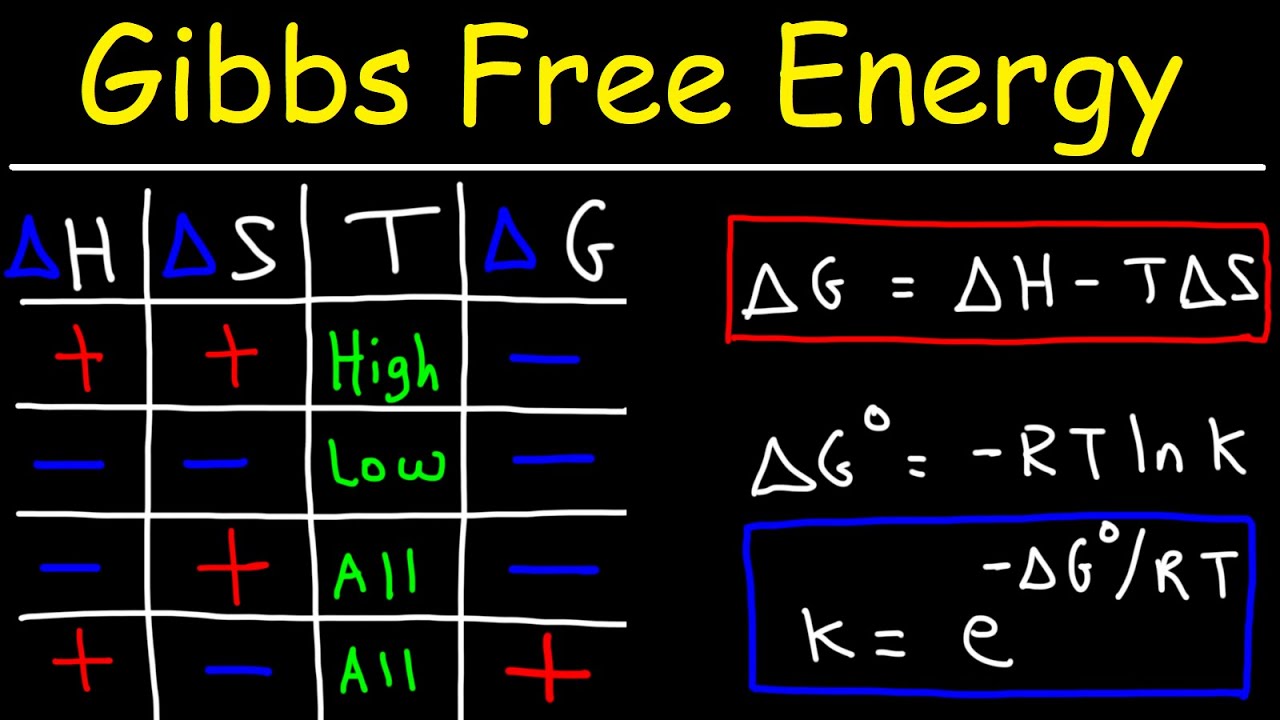
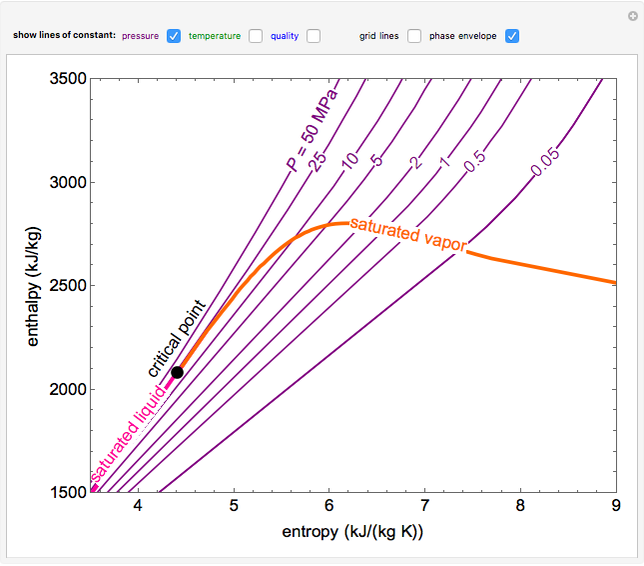
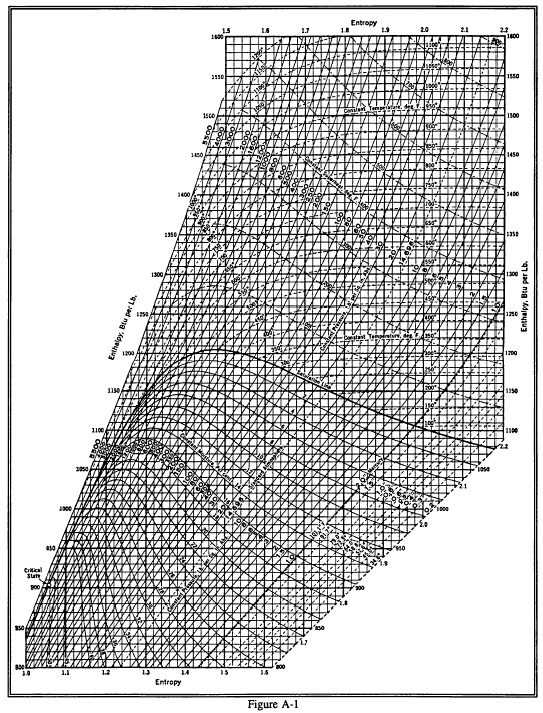
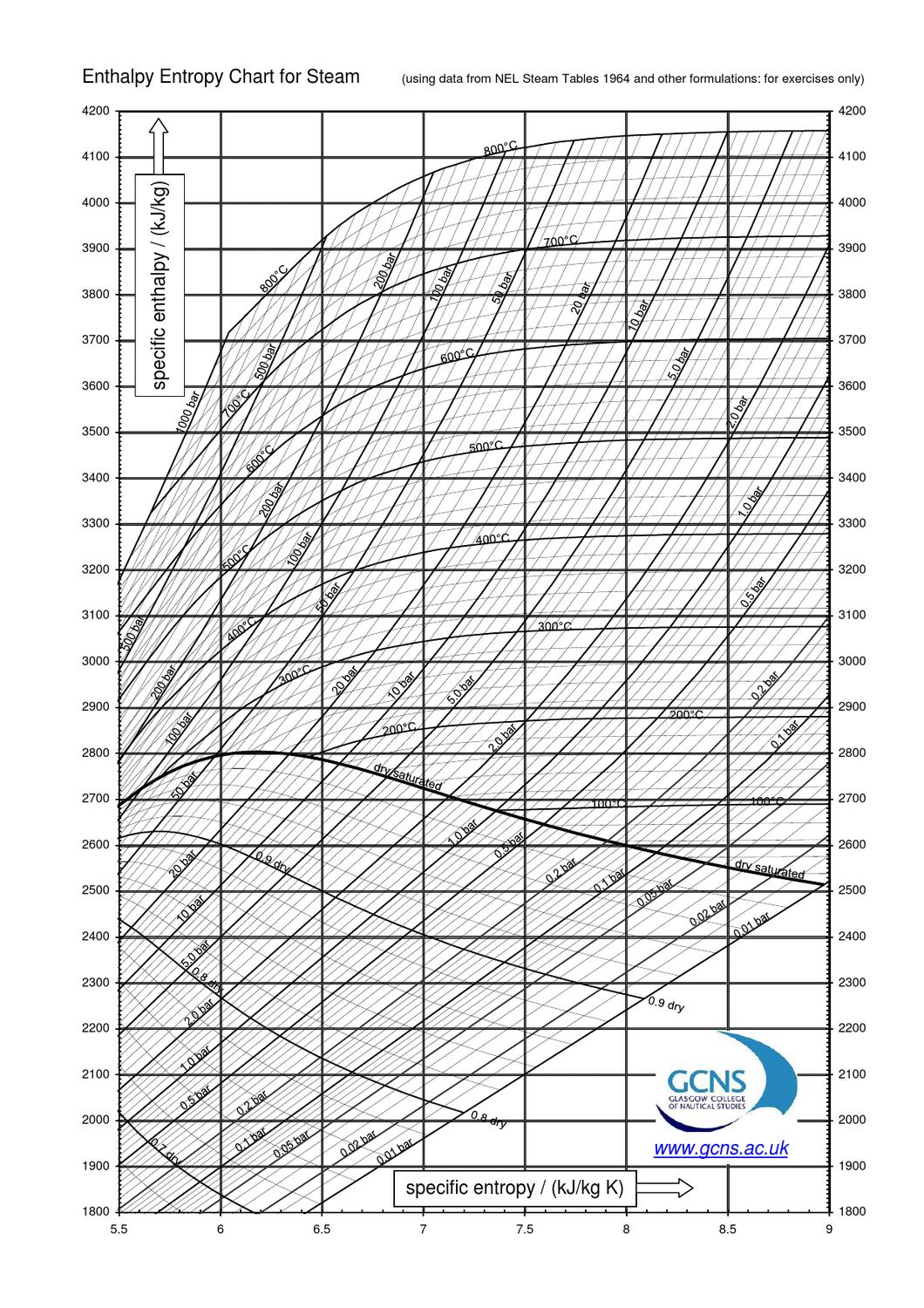
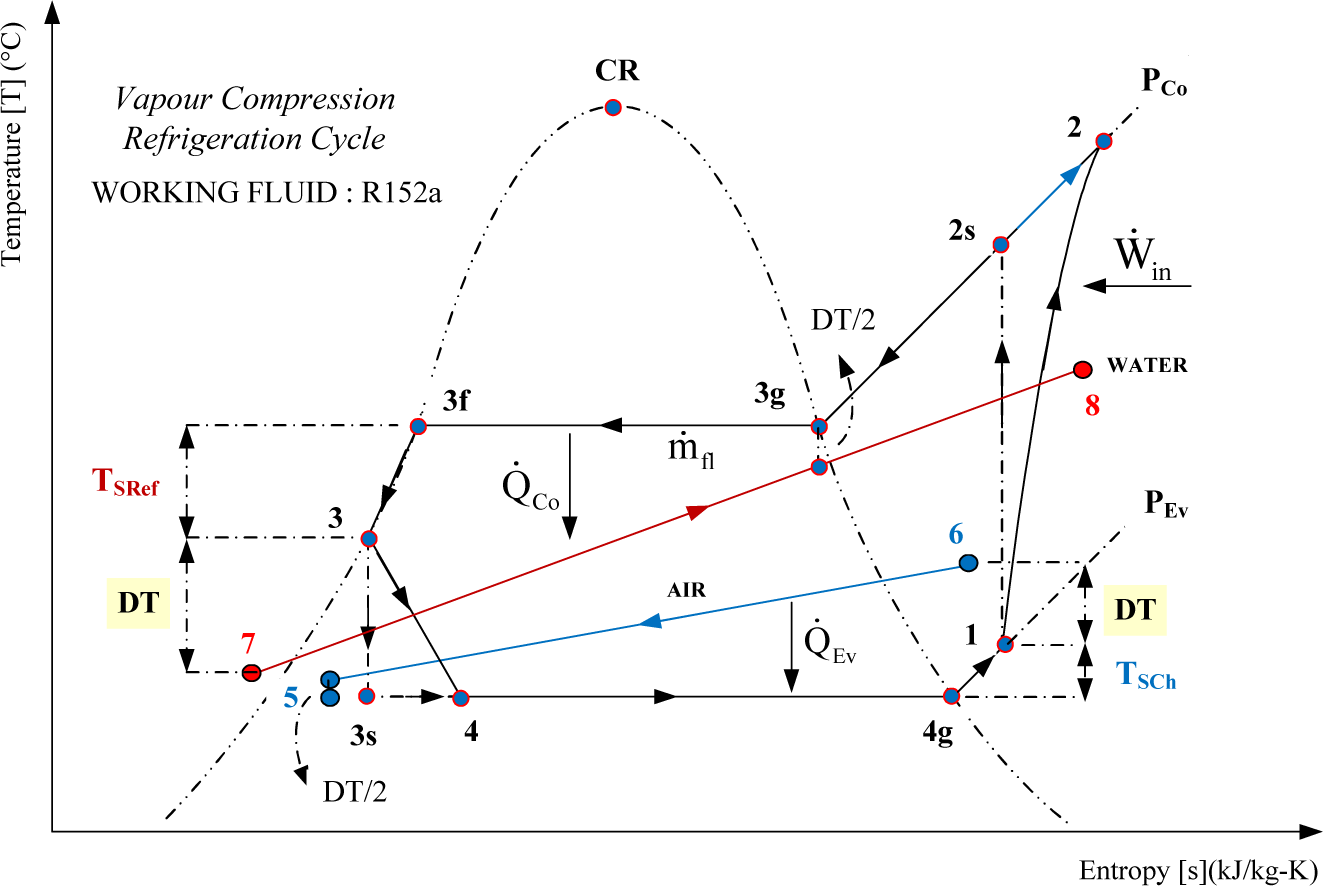
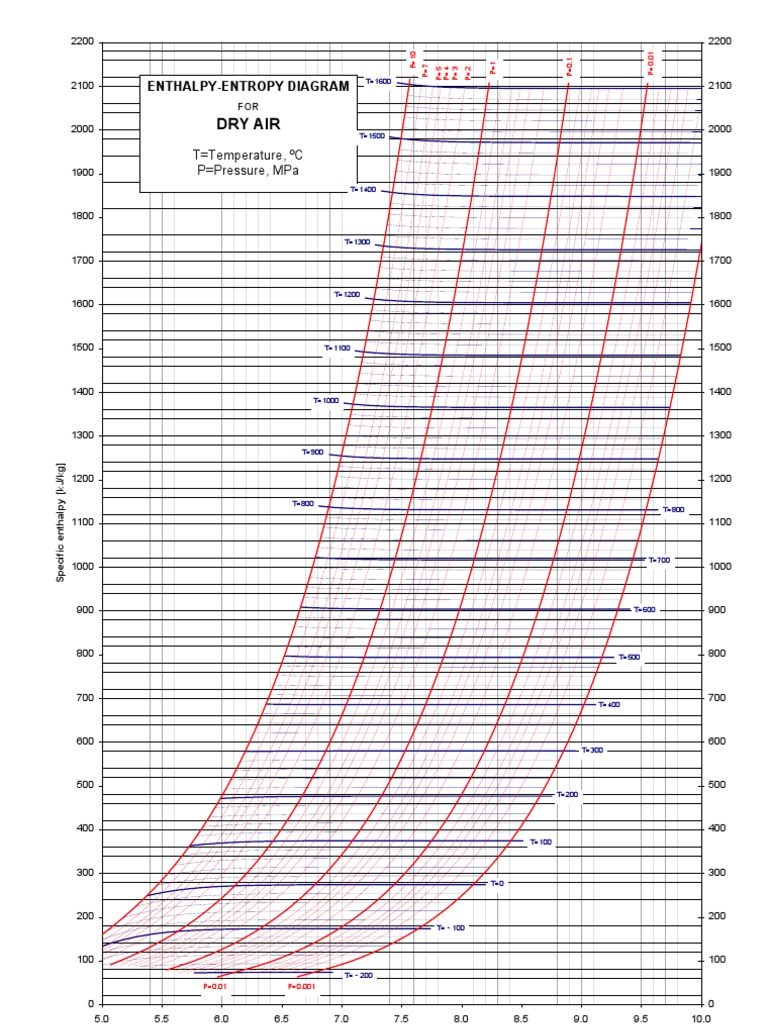
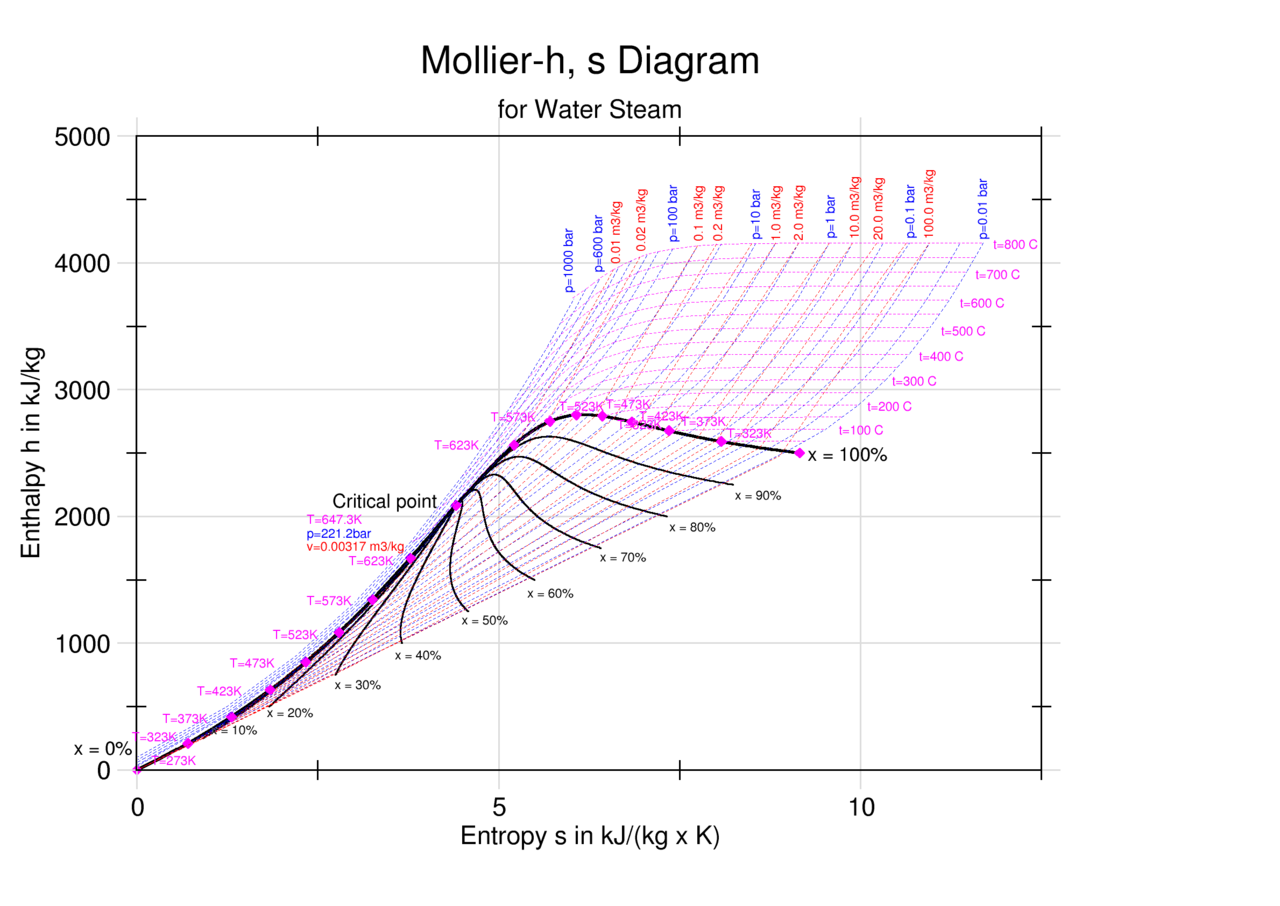
Web the mollier diagram is a graph used in thermodynamics to visualize the relationships between temperature, pressure, specific volume, enthalpy, and entropy of. It is a state function in thermodynamics used in. The symbol for enthalpy is “h.” enthalpy is also considered to be the sum of. The mollier diagram is useful. Web where enthalpy is a measurement of energy potential, entropy measures the randomness of energy with relation to heat. What is the enthalpy change? When energy is added) or decreases. Web the heat that passes into or out of the system during a reaction is the enthalpy change. New york, new york, 1979; S=0 kj/k*kg and h=0 kj/kg. Go to standard state and standard enthalpy of formation for. Calculate propierties of wet, saturated and superheated steam, steam quality and more. Web standardized enthalpies and entropies of some common substances: Some important terms related to enthalpy: Web the figures and tables below shows how water enthalpy and entropy changes with temperature (°c and °f) at water saturation pressure (which for practicle use, gives the.
Whether The Enthalpy Of The System Increases (I.e.
Web definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well. Equilibrium states at 25oc (77of) and 1 atm. Web enthalpy ( / ˈɛnθəlpi / ⓘ) is the sum of a thermodynamic system 's internal energy and the product of its pressure and volume. Web the figures and tables below shows how water enthalpy and entropy changes with temperature (°c and °f) at water saturation pressure (which for practicle use, gives the.
Some Important Terms Related To Enthalpy:
The mollier diagram is useful. Understanding enthalpy and entropy clarify what these. U = specific internal energy. What is the enthalpy change?
Web The Mollier Diagram Is A Chart On Which Enthalpy (H) Versus Entropy (S) Is Plotted.
Web the heat that passes into or out of the system during a reaction is the enthalpy change. Web steam tables online calculator, completely free! Enthalpy is defined as the total heat content or total useful energy of a substance. New york, new york, 1979;
The Messy Room On The Right Has More.
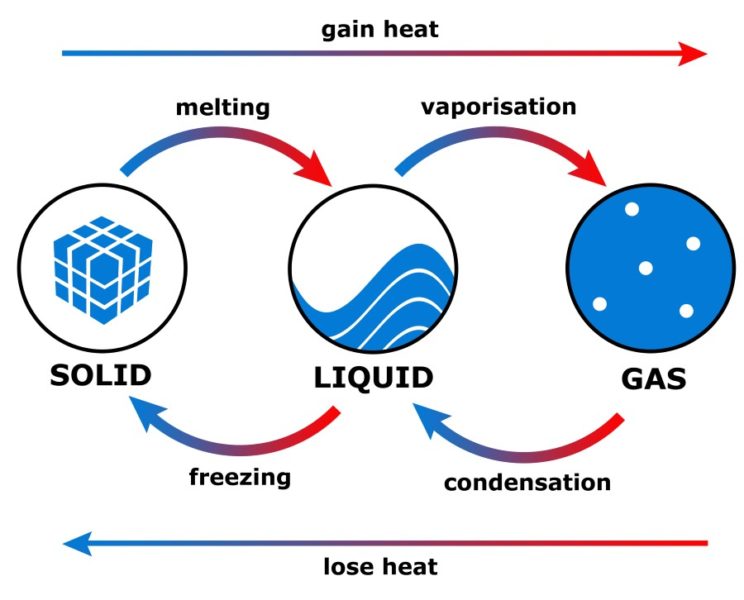
Web where enthalpy is a measurement of energy potential, entropy measures the randomness of energy with relation to heat. When energy is added) or decreases. Web based on the phase diagram (supplementary fig. The symbol for enthalpy is “h.” enthalpy is also considered to be the sum of.