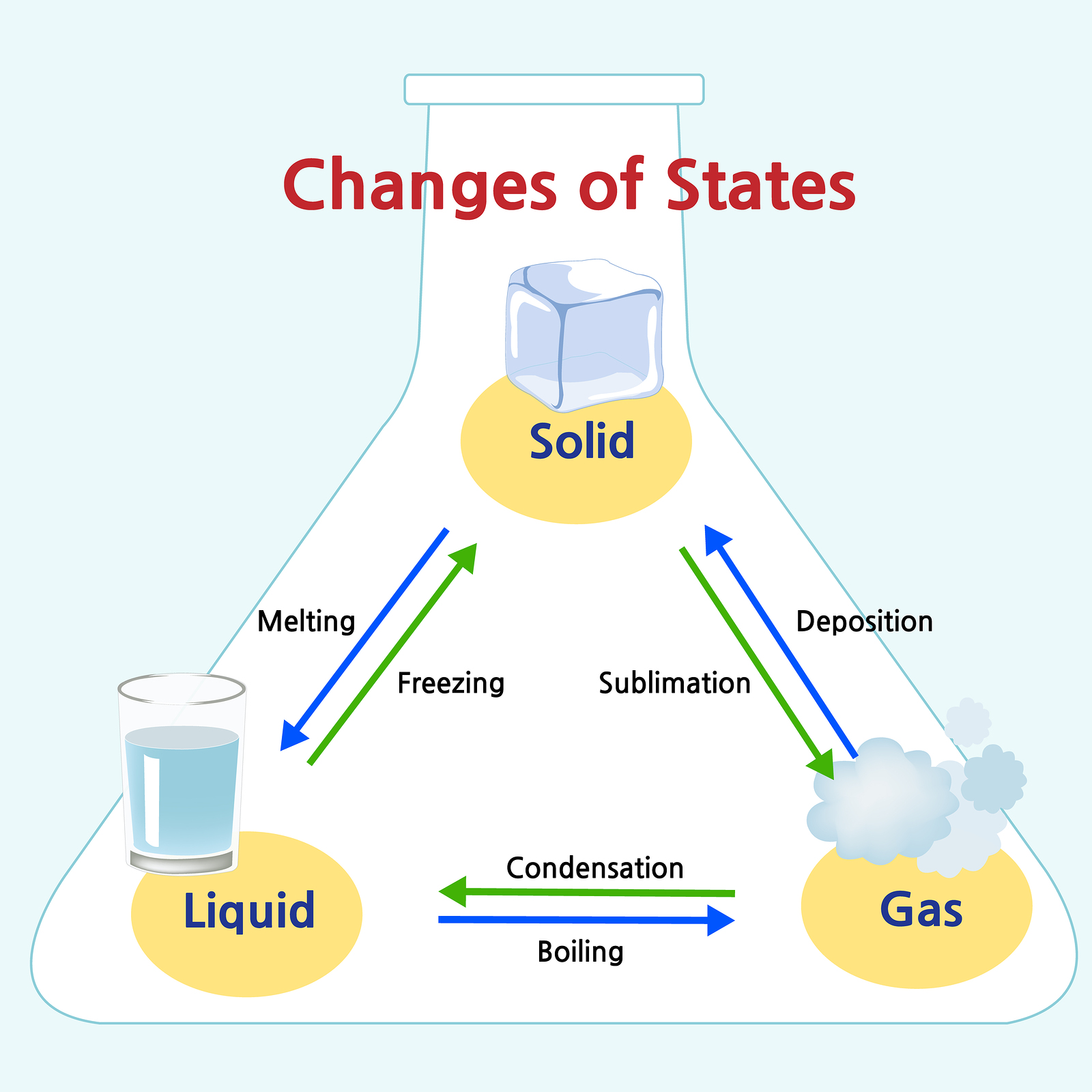
Solid are tightly packed, usually in a regular pattern. Web aligned with the topic properties of the three states of matter, the chart here stimulates interest, summarizes the properties of solids, liquids and gases and assists in distinguishing between them. Where solids and liquids differ is in their shape. Web this is another great question. In this state, the distinction between liquid and gas disappears.
The particles vibrate back and forth within their fixed positions and do not move freely. Where solids and liquids differ is in their shape. Gas can be compressed much more easily than a liquid or solid. Highly strong intermolecular forces between the molecules, leads to a definite volume in solids. Watch different types of molecules form a solid, liquid, or gas.
Gas vibrate and move freely at high speeds. Web gas are well separated with no regular arrangement. Liquid are close together with no regular arrangement. Unlike a liquid, a gas easily expands or contracts to fill the entire volume of the container. Web reimagine the everyday with a closer look at the states of matter!
Relate the interaction potential to the forces. Quartz (solid), water (liquid), nitrogen dioxide (gas). Web aligned with the topic properties of the three states of matter, the chart here stimulates interest, summarizes the properties of solids, liquids and gases and assists in distinguishing between them. Liquid are close together with no regular arrangement. Solid vibrate (jiggle) but generally do not move from place to place. In the video here, sal uses a horizontal line through the phase diagram. Web compare three states of matter: Matter is usually classified into three classical states. Web solid refers to a form of matter which has structural rigidity and has a firm shape which cannot be changed easily. Web difference between solid liquid and gases; Some substances exist as gases at room. The intermolecular forces are stronger than gases but weaker than solids. In this state, the distinction between liquid and gas disappears. Expand a little when heated. Web examples of liquids include water, juice, and vegetable oil.
The Particles Vibrate Back And Forth Within Their Fixed Positions And Do Not Move Freely.
Web compare three states of matter: Web a vapor can exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure of the liquid (or solid). As long as you are at 100 c, you can change the phase by changing the pressure on the system. Where solids and liquids differ is in their shape.
Direct The Children Of Grade 2 And Grade 3 To Observe The Illustrations Given In This Circle.
Solid vibrate (jiggle) but generally do not move from place to place. A gas will fill any container, but if the container is not sealed, the gas will escape. Highly strong intermolecular forces between the molecules, leads to a definite volume in solids. In this state, the distinction between liquid and gas disappears.
Matter Occurs In Four States:
Web this vapor is still h 2 o, just in gas form. Solids are incompressible and have high density, compared to liquids and gases. They vibrate and move freely at high speeds. Solid are tightly packed, usually in a regular pattern.
Solid (The Ice), Liquid (The Water) And Gas (The Vapor) Are The Three Most Common States Of Matter — At Least On Earth.
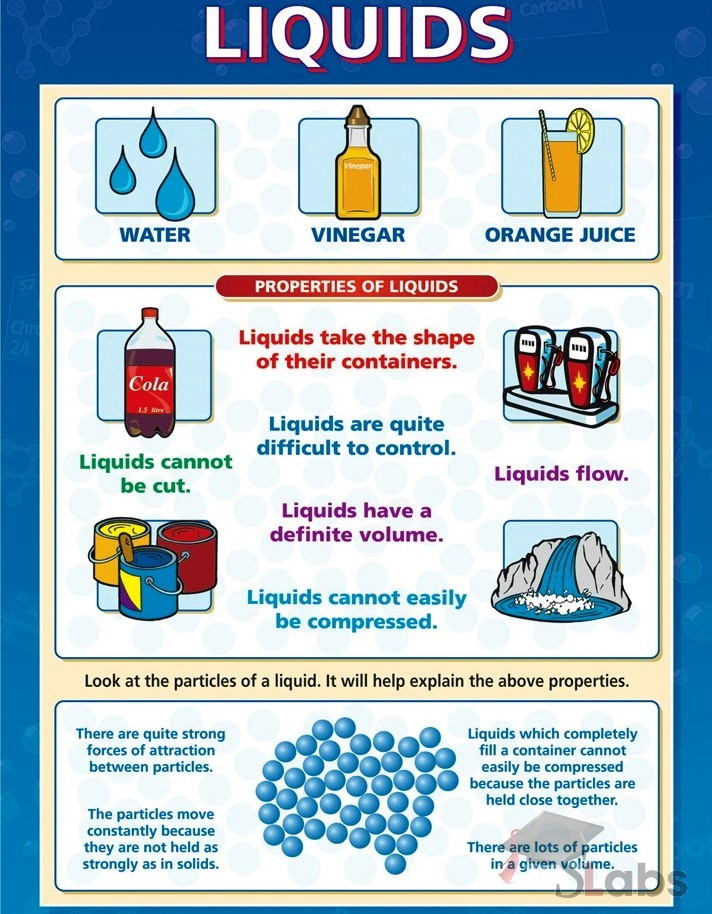
Web they don’t pour like a liquid. Liquid is a substance, that flows freely, having a definite volume but no permanent shape. Updated on june 07, 2024. Web the atoms and molecules in gases are much more spread out than in solids or liquids.