Web solid is the state in which matter maintains a fixed volume and shape, liquid is the state in which matter adapts to the shape of its container but varies only slightly in volume, and gas is the state in which matter expands to occupy the volume and shape of its container. While at temperatures above \ (100^\text {o} \text {c}\), water is a gas (steam). At low temperatures (below \ (0^\text {o} \text {c}\)), it is a solid. Solids and liquids have a fair bit in common, as in both states the molecules are joined together. Web watch different types of molecules form a solid, liquid, or gas.
Expand a little when heated. Also included is a description on how the molecules for each move and if they have a definite shape or volume. Web you can use this handy chart to compare the features of the three states of matter: At low temperatures (below \ (0^\text {o} \text {c}\)), it is a solid. Web watch different types of molecules form a solid, liquid, or gas.
A gas lacks either a defined shape or volume. Expand a little when heated. Where solids and liquids differ is in their shape. Web solid (the ice), liquid (the water) and gas (the vapor) are the three most common states of matter — at least on earth. In ancient greece, one philosopher recognized how water could change form and reasoned that everything must be.
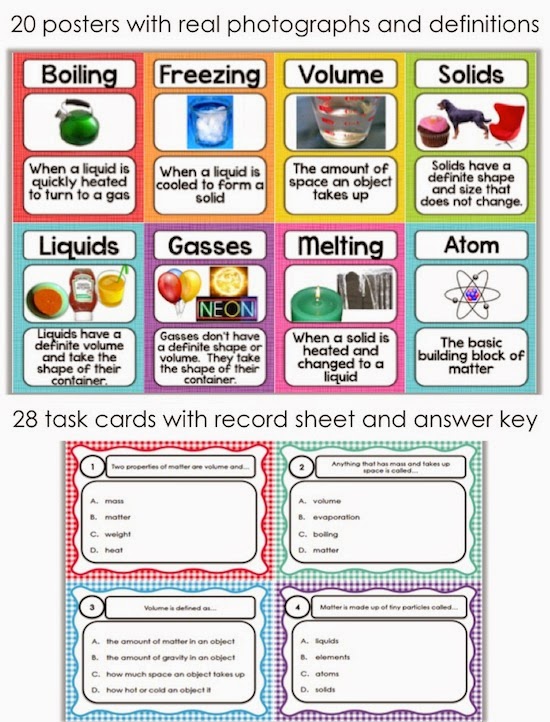
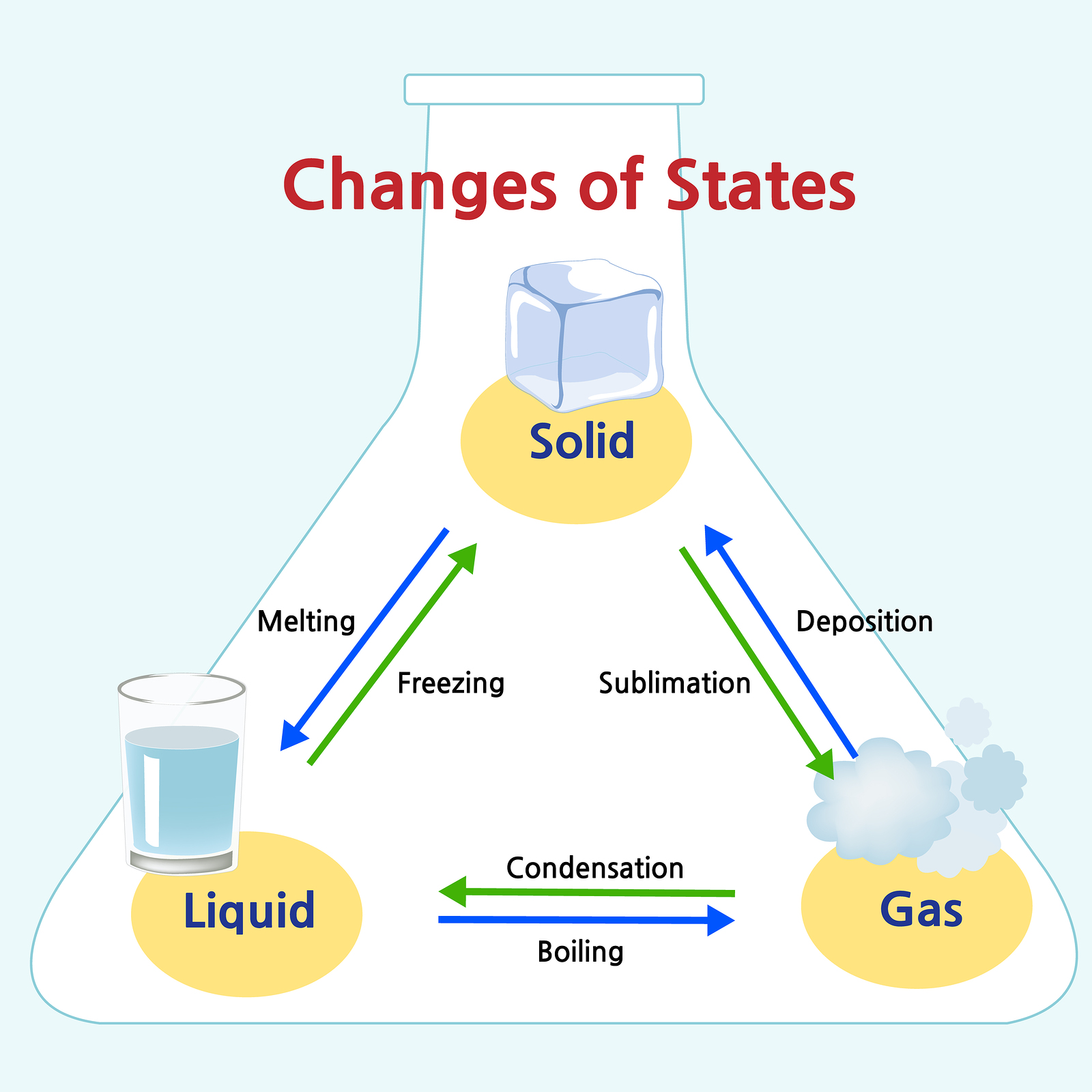
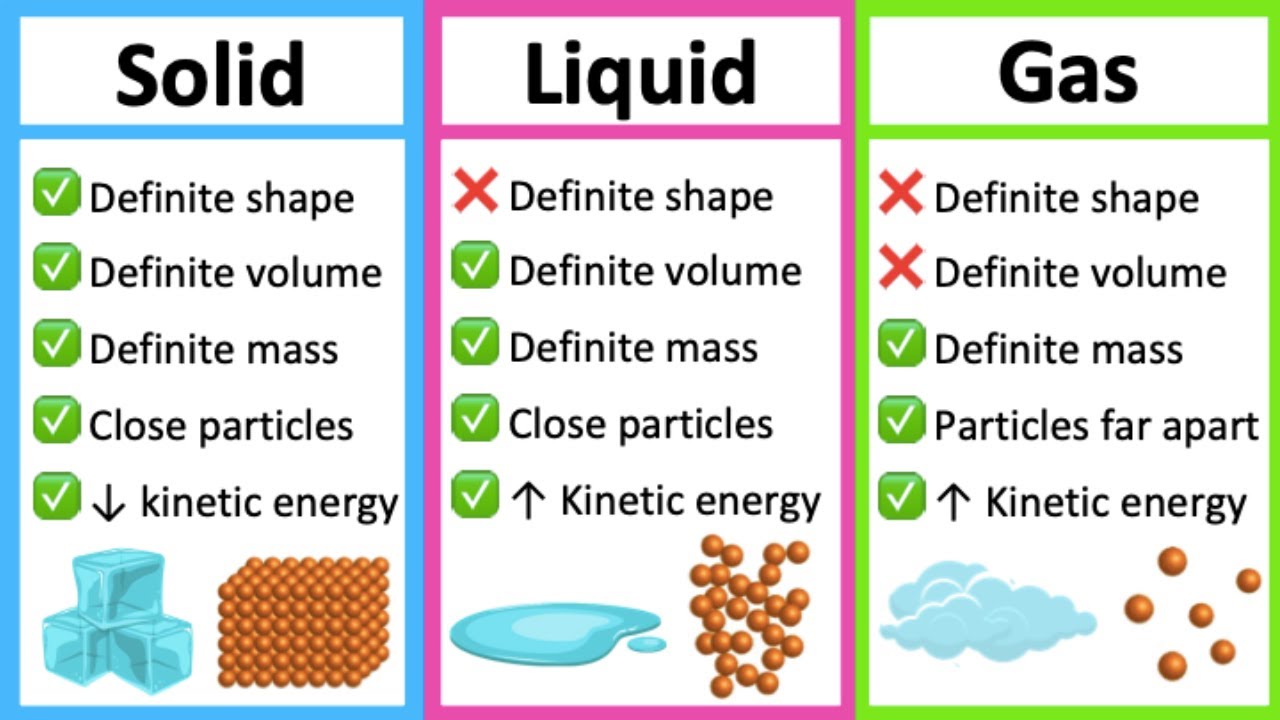
Liquids have a definite volume, but take the shape of the container. Add or remove heat and watch the phase change. Where solids and liquids differ is in their shape. Visit byju’s to learn more about it. A solid has a definite shape and volume. Expand a little when heated. Solids have a definite shape and volume. Gases have no definite shape or volume. Expand a little when heated. For example, if i told you that i was at 0 degrees, let's say 0 degrees is right there, if i'm at 0 degrees celsius and 1 atmosphere, where am i? No expand when heated expand greatly when heated. Also included is a description on how the molecules for each move and if they have a definite shape or volume. A gas lacks either a defined shape or volume. Gases, on the other hand, have uniquely different properties compared to solids and liquids. Web this matter anchor chart /poster will help your students learn about the phases of matter and give them information about the properties of solids, liquids and gases.
At Low Temperatures (Below \ (0^\Text {O} \Text {C}\)), It Is A Solid.
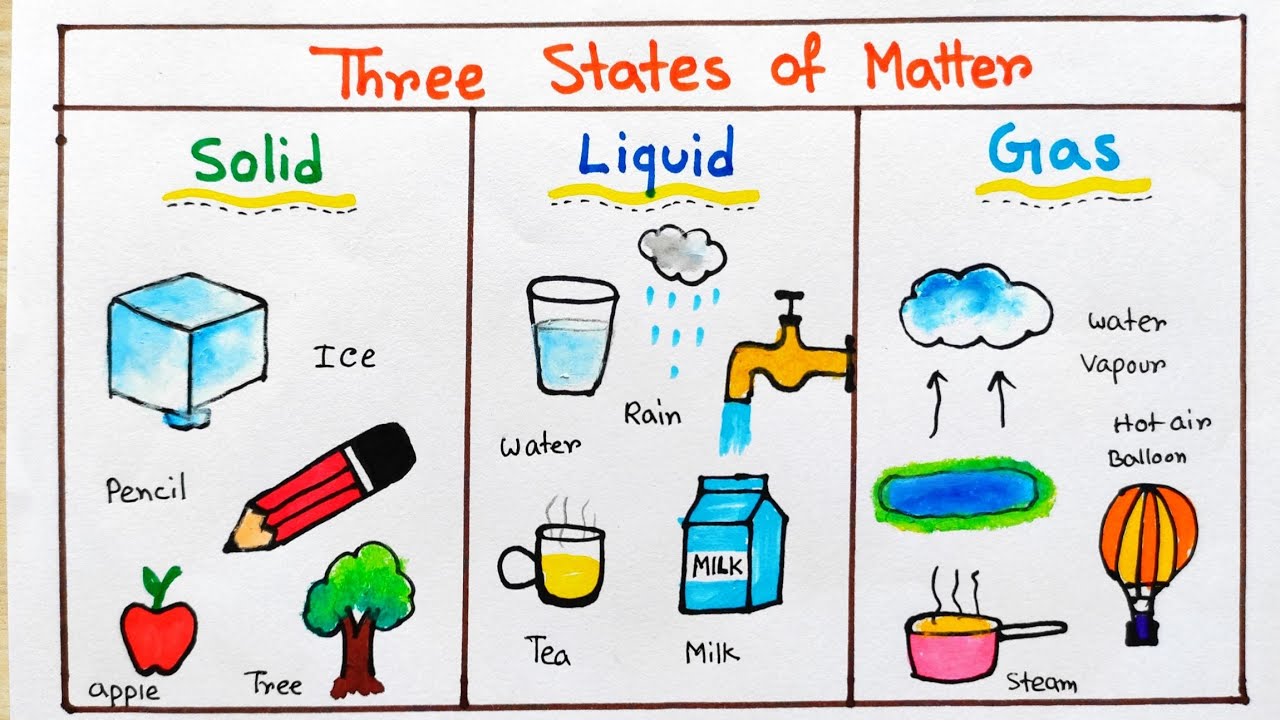
Web this anchor chart for students features a chart with different examples for solid liquid and gas. Web solid (the ice), liquid (the water) and gas (the vapor) are the three most common states of matter — at least on earth. It is important to know the major differences between solids, liquids and gases. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation.
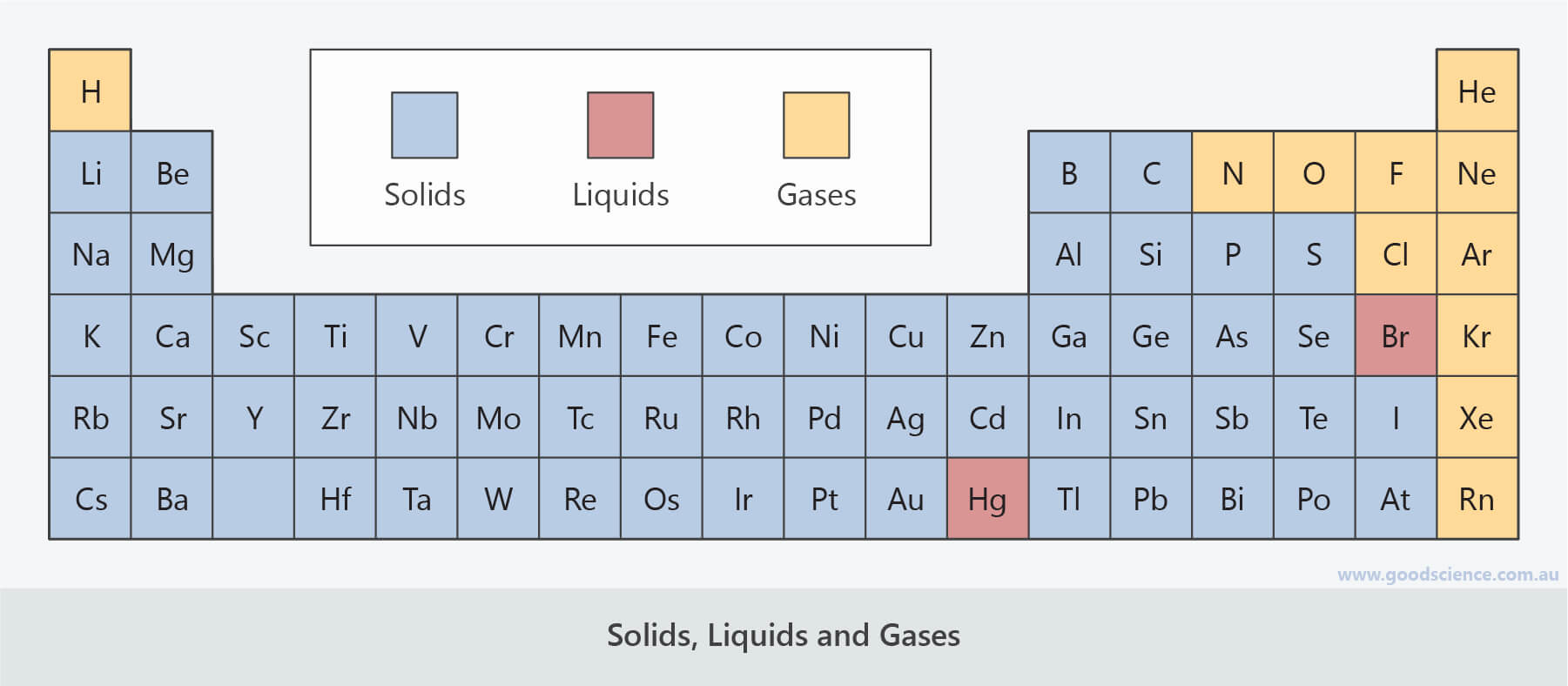
Web Water Is The Only Common Substance That Is Naturally Found As A Solid, Liquid Or Gas.
Solids and liquids have a fair bit in common, as in both states the molecules are joined together. Web aligned with the topic properties of the three states of matter, the chart here stimulates interest, summarizes the properties of solids, liquids and gases and assists in distinguishing between them. Solids, liquids and gases are known as states of matter. Under exceptional conditions, other states of matter also exist.
Expand A Little When Heated.
Web liquids and solids are often referred to as condensed phases because the particles are very close together. Included is the freezing point and melting point of water. Before we look at why things are called solids, liquids or gases, we need to know more about matter. Web solids, liquids, and gases are the three primary states of matter.
Add Or Remove Heat And Watch The Phase Change.
Web the four main states of matter are solids, liquids, gases, and plasma. Web watch different types of molecules form a solid, liquid, or gas. Solids and liquids have a fair bit in common, as in both states the molecules are joined together. Molecular arrangement of solids is regular and close, but liquids have irregular and sparse molecular arrangement and gases, too have random and.