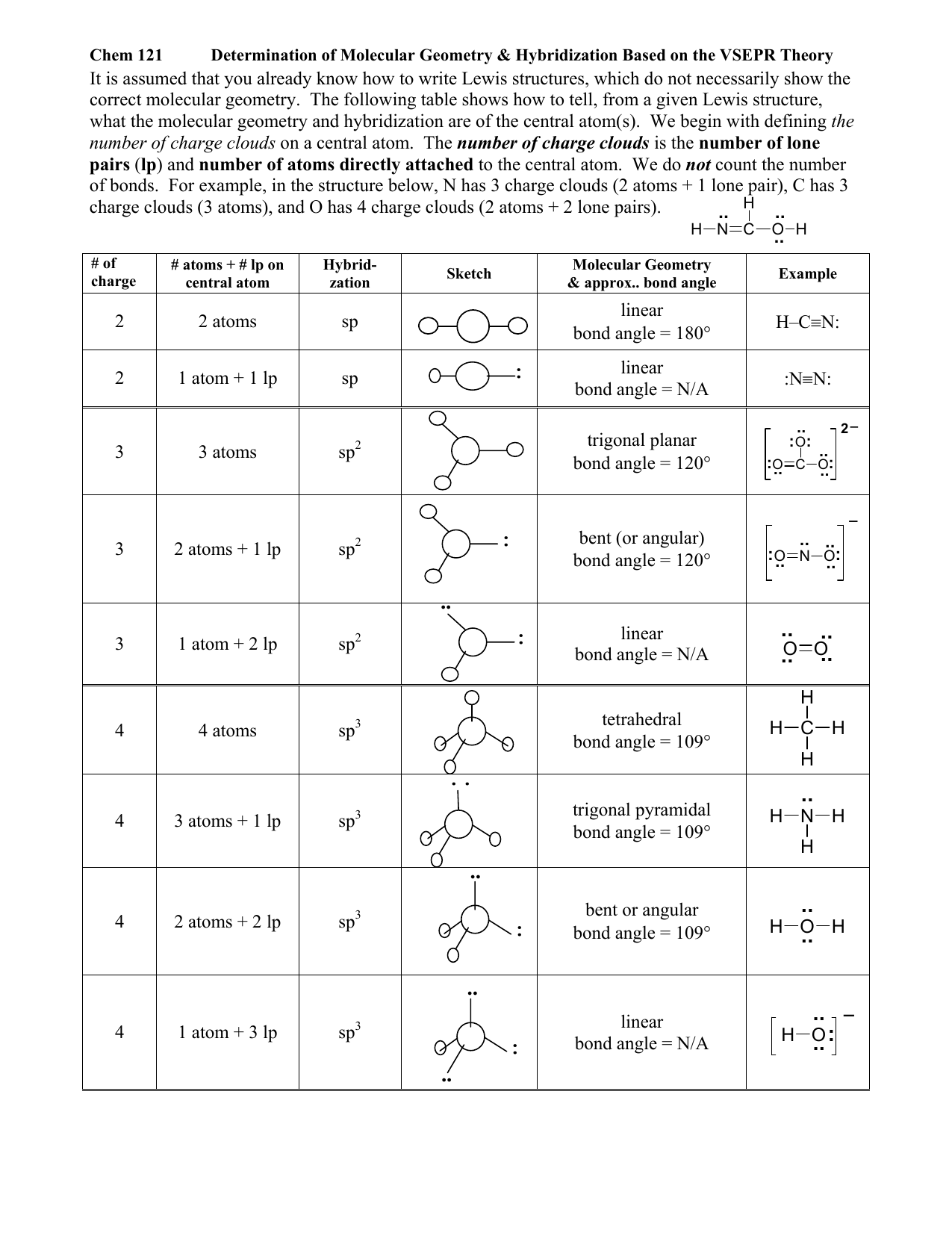
Lewis structures provide us with the number and types of bonds around a central atom, as well as any nb electron pairs. Usually, the electrons in the outermost shell of an atom, also known as valence electrons, take part in chemical. Describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule.
Overlap of atomic orbitals with other atomic orbitals (bonds in. This 109.5 o arrangement gives tetrahedral geometry (figure 4). Web hybridization of an s orbital with all three p orbitals (p x , p y, and p z) results in four sp 3 hybrid orbitals. Now to understand this we need to know the steps to draw a lewis structure at first. Want to join the conversation?
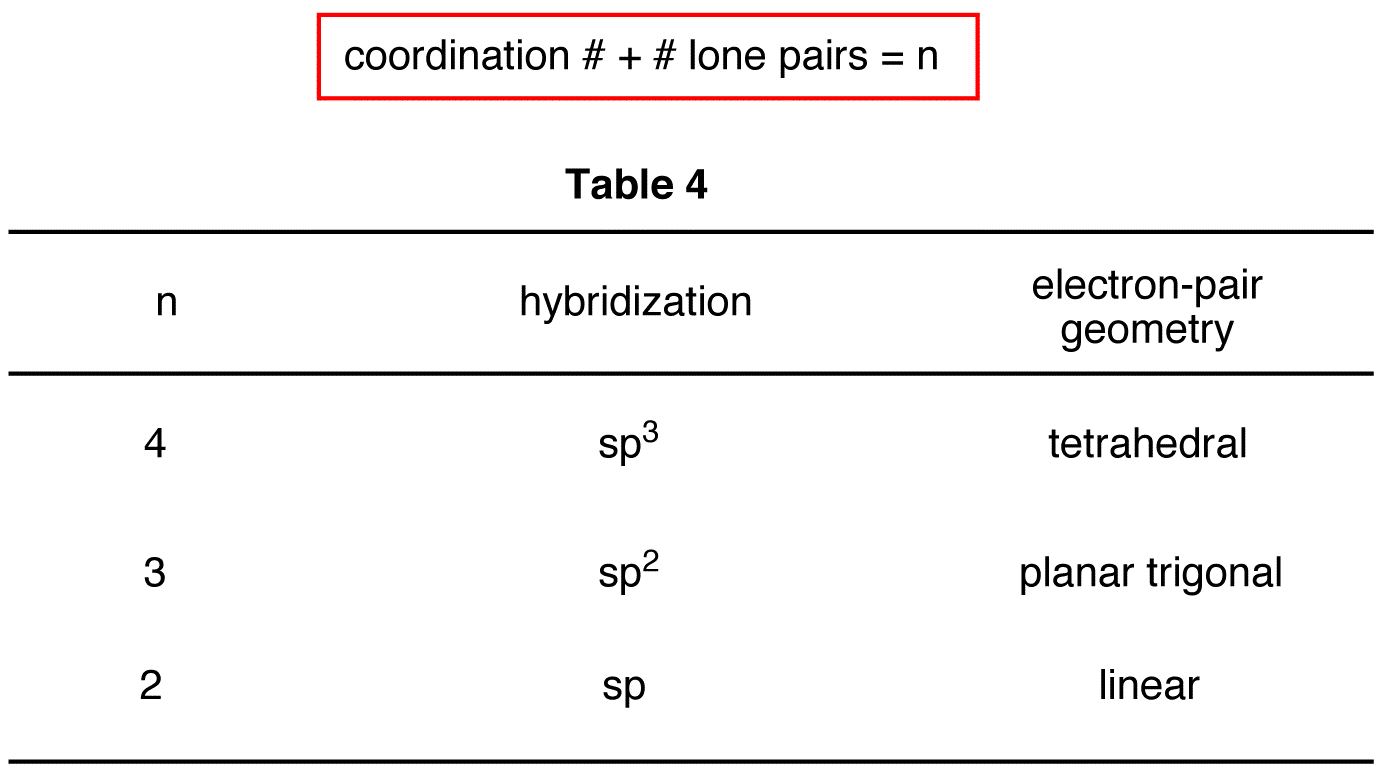
The steric number is how many atoms are bonded to a central atom of a molecule plus the number of lone electron pairs attached to that atom. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. Web hybridization of an s orbital with all three p orbitals (p x , p y, and p z) results in four sp 3 hybrid orbitals. Predict the actual geometry of the molecule or ion ( 6 ). Web as we’ve seen, the ideal geometry for arranging four pairs of electrons is tetrahedral, which makes the hybridization of the central atom sp 3.
Web board index chem 14a molecular shape and structure determining molecular shape (vsepr) Reactions involve making and breaking of bonds! Now to understand this we need to know the steps to draw a lewis structure at first. This leaves the molecule with a “piano stool” arrangement of atoms about the central atom, which we call “trigonal pyramidal” geometry. First and foremost it is important to determine how many valence electrons are present in the compound. Web this vsepr chart shows you all of the common vsepr geometries, organized by the steric number and how many lone electron pairs they have. Orbitals are combined in order to spread out electrons. Web as we’ve seen, the ideal geometry for arranging four pairs of electrons is tetrahedral, which makes the hybridization of the central atom sp 3. This 109.5 o arrangement gives tetrahedral geometry (figure 4). Do you know that several factors make atoms come together and combine to form several different chemical compounds? Want to join the conversation? Web in sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. Usually, the electrons in the outermost shell of an atom, also known as valence electrons, take part in chemical. Covalent bonds are formed by: Then, compare the model to real molecules!
Web Predict The Electronic Geometry Using All Areas Of Electron Density (Or, Effective Electron Pairs) And The Ideal Bond Angles Associated With This Geometry ( 5 ).
First and foremost it is important to determine how many valence electrons are present in the compound. Web hybridization increases the overlap of bonding orbitals and explains the molecular geometries of many species whose geometry cannot be explained using a vsepr approach. The angle between adjacent bonds of an atom. Determine the hybridization of the central atom ( 7 ).
Web Board Index Chem 14A Molecular Shape And Structure Determining Molecular Shape (Vsepr)
Lewis structures provide us with the number and types of bonds around a central atom, as well as any nb electron pairs. The following table shows the equivalence: We will illustrate the use of this procedure with several examples, beginning with atoms with two electron groups. Web hybridization directly correlates to molecular geometry!
Sp 3 Hybrid Orbitals Are Oriented At Bond Angle Of 109.5 O From Each Other.
In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Want to join the conversation? Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. When atoms come together and create bonds, a new molecule is created.
This 109.5 O Arrangement Gives Tetrahedral Geometry (Figure 4).
Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. In this compound valence electron will be as following: Predicted bond angle(s) hybridization of central atom. Web explore molecule shapes by building molecules in 3d!





2]%2B%2B4.%2BTetrahedral.%2Bsp3.%2B[Cd(NH3)4]2%2B.jpg)